How to prescribe Triumeq?
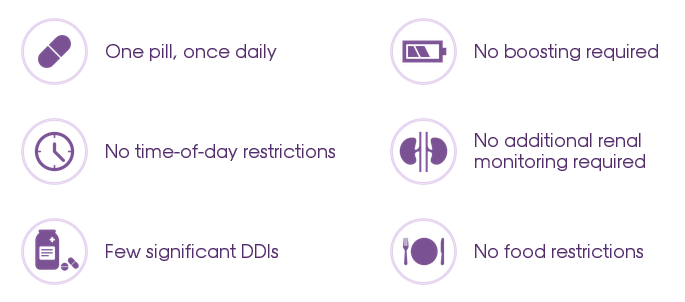
Once-daily TRIUMEQ makes dosing and administration simple for your patients
TRIUMEQ (dolutegravir/abacavir/lamivudine) is indicated for the treatment of Human Immunodeficiency Virus (HIV) infected adults and adolescents above 12 years of age weighing at least 40 kg. 1
Dosing recommendation 1

Before initiating treatment with abacavir-containing products, screening for carriage of the HLA-B*5701 allele should be performed in any HIV-infected patient. Abacavir should not be used in patients known to carry the HLA-B*5701 allele. [1] Find out more on HLA-B*5701 screening here.
Contraindications 1
Hypersensitivity to dolutegravir, abacavir or lamivudine or to any of the excipients. Co-administration with dofetilide.
For further information, please see Triumeq prescribing information
▼This medicinal product is subject to additional monitoring.
*TRIUMEQ is a fixed-dose pill and should not be prescribed for patients requiring dose adjustments. Separate preparations of dolutegravir, abacavir, and lamivudine are available in cases where discontinuation or dose adjustment is required. 1
†TRIUMEQ is not recommended for patients with integrase inhibitor resistance.* 1
‡TRIUMEQ is not recommended for co-administration with efavirenz, nevirapine, rifampicin, or tipranavir/ritonavir. 1
*See How to prescribe Tivicay? for recommended dolutegravir dosing in patients with resistance to the integrase class.
TRIUMEQ is a registered trademark of the ViiV Healthcare group of companies
Atripla is a registered trademark of Bristol-Myers Squibb & Gilead Sciences, LLC.






